Plant Tissue Culture is a process of growing isolated plant cells or organs in an artificial nutrient media outside the parent organism.
In other words, it is an in vitro culture of plant cells or tissues on an artificial nutrient media under aseptic conditions in glass containers.
This is a technique by which new plants can be raised using plant parts or cells. These small parts can be pollen, leaves, seed, root tip, embryos, etc.
Since all the above organs or cells contain the same genetic material as parent plants, a new plant of the same variety can be grown.
This capacity of plant parts or cells to grow into a full plant is termed “totipotency.”
Media for tissue culture

Nutrient media plays an important role in tissue culture.
It is vital for the proper and timely growth of cells and their multiplication.
Since nutrient media is the only source of nutrition, it should supply all the basic requirements.
These include carbohydrates, amino acids, minerals, hormones, salts, etc. at proper proportions.
It should be sterile and non-toxic to the tissue or cell under culture.
All the media ingredients are to be sterile hence one can use autoclave or membrane filters based on their thermal resistance power. Tissue culture media preparation should be done in aseptic rooms and conditions.
Inorganic elements
Macroelements
| Macroelement | Sources |
|---|---|
| Potassium supplement | KCl, KNO3, KH2PO4 |
| Calcium supplement | CaCl2.2H2O, Ca (NO3)2. 4H2O |
| Magnesium | MgSO4 |
| Phosphorous | NaH2PO4.H2O, KH2PO4, NaCl |
Microelements
| Microelement | Source |
|---|---|
| Manganese | MnCl2 |
| Zinc | ZnSO4 |
| Copper | CuSO4 |
| Aluminum | AlCl3 |
Other micronutrients like bismuth, molybdenum, and nickel are also added.
Organic compounds
Carbon source– Sucrose/glucose 2-4%
Vitamin source
Vitamins have diverse roles in enhancement of growth. They act as co-enzymes, hormones and regulators of cell physiology. Some essential vitamins required for tissuue culture include.
| Vitamin | Quantity |
|---|---|
| Thiamin HCl | 0.01mg/lit |
| Pyridoxine | 0.5 mg/lit |
| Nicotinic acid | 0.5 mg/lit |
| Folic acid | 0.5 mg/lit |
| Pantothenic acid | 0.5 mg/lit |
Amino acid sources
Arginine, aspartic acid, glutamic acid, glutamine, methionine.
Growth hormones
- Indole-3 Acetic acid.
- Naphthalene acetic acid.
- 2, 4-Dichloro phenoxy acetic acid (2,4 D).
- Kinetin, zeatin, benzyl adenine.
Coconut water, yeast extract, malt extract, and casein hydrolysate.
Also one can include activated charcoal to adsorb impurities from media. A pH of 5.2 to 5.6 is best, with a temperature of 25 degrees c.
Tissue culture equipment like a Complete air-conditioned lab, laminar airflow, autoclave, BID incubators, and Shakers are also needed.
You can find more at Plant Tissue Culture: Techniques.
Steps in tissue culture
1. Tissue or cell of an interesting plant is selected and sterilized (disinfected) by mercuric chloride or alcohol.
Sterilization of cells
The cells taken for tissue culture are to be surface sterilized. This helps the cell wall and tissue surfaces free from bacterial or fungal infections. Care should be taken during their handling, transfer, etc. to keep them free from infection.
2. Then, the tissue is placed in media in a conical or volumetric flask and incubated with a proper oxygen supply and the right temperature.
Oxygen supply
Since tissue has no direct mechanism to take up oxygen, an oxygen supply has to be provided. The gas should be free from contamination and also aseptic. The gas flow rate and pressure in the tissue culture chamber should be optimal.
3. The tissue or cell multiplies and then forms plant lets.
4. This can be transplanted into the greenhouse. Tissue culture plants are highly sensitive to tolerate natural environmental conditions. They have to be slowly adapted to a normal atmosphere. So first they are to be grown in greenhouses.
Tissue growth curve
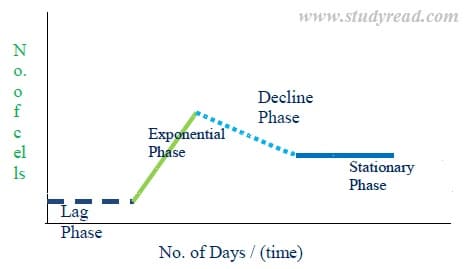
When a cell or tissue is incubated in nutrient media, it shows a phase difference in growth.
There are four phases of tissue growth, and when a graph is plotted with growth versus curve, we obtain a tissue growth curve. This growth curve has
a) Lag phase
This is the first phase of the cell or tissue in the artificial nutritional media. As you can see in the above graph, the phase shows a horizontal line parallel to X-axis.
In this phase, the cell or tissue tries to adapt and get adjusted to the new environment out of the mother plant. Here the cells grow in size but don’t multiply.
Hence, tissue weight slightly increases, but the number of cells in the media is the same.
b) Exponential phase (log phase)
- Here the cells multiply profusely and grow in numbers. The line in the graph shows a prominent rise from the lag phase.
- In this phase, the physical size of the callus (mass of the tissue) in the media grows enormously.
- This is a useful phase as the desired bi-products are produced in large quantities in the media.
c) Decline phase
- The curve suddenly shows a decline in the graph of tissue growth. In this phase, the nutrients in the media are exhausted.
- Due to this, the multiplication of cells slows down.
- The size of the callus to starts to decrease to the previous phase. Even the production of bioproducts decreases.
d) Stagnant phase
- This is the final phase of tissue culture. The curve in the graph shows a straight horizontal line.
- The cells here remain in fewer numbers without further multiplication. This is due to the lack of nutrients, accumulation of toxins, etc.
- The decline and stagnant phases can be seen in batch cultures but not in continuous cultures.
Plant Tissue Culture Techniques
There are mainly two major techniques in plant tissue culture.
a) Static culture (Solid-agar Medium)
- It can also be called callus plant tissue culture.
- In this procedure, the plant tissue is grown on a solid agar medium and always gives rise to a tissue mass called a callus.
- This callus culture technique is easier as it is easier and even more convenient for the initial maintenance of cell lines and also for carrying out the investigation studies related to organogenesis, i.e., organ formation.
b) Suspension cultures (Liquid media)
- Here the cell aggregates or even single cells are grown in liquid culture.
- The cells are kept suspended by using agitators/shakers/ impellers.
- The actual growth rate of the liquid-suspension cultures is much higher than those grown in a solid-agar medium.
- Besides, this technique provides superior control over biomass growth as the nutrient medium constantly surrounds the cells.
For more techniques, you can read the book on Plant Tissue Culture.
Types of suspension culture:
1) Batch suspension cell culture
- Here the cells or tissues are grown in a fixed volume of nutrient medium.
- Once the cells reach the exponential phase, the entire culture is replaced with a new one.
- It is a closed type of culture.
2) Continuous suspension culture
- Here there is the continuous addition of nutrition media.
- The dilution rate is such that an equivalent volume of media is removed out proportional to the inflow from the top.
- The cells are always kept in the exponential growth phase.
I) Open type: Here the system is kept continuous with constant addition and removal of cultured cells.
II) Closed type: Here cell proliferates till the completion of the exponential phase. Then there is a fresh addition of nutrient media & culture media.

Dear sir/mam.
Thank you for the detailed, very important knowledge about this
specific subject.
Best regards.
Please help me with incubation of culture with diagram
Thanks for giving me new knowledge. I hope I can learn a lot thing from you especially in tissue culture techniques.
Extremely informative,would like to know the development of a green house,sterilization,etc.,because I am quite new to this.
Thanks a lot….. It is very informative
I get it helpful.
THANKS A LOT