Atomic absorption spectroscopy (AAS) is another interesting type of spectroscopy.
It is specifically designed for the analysis of the metals and metalloids substances.
By definition, AAS is a quantitative analytical technique wherein the absorption of a specific wavelength of radiation by the neutral atoms in the ground state is measured.
The more the number of the atoms in a given sample, the higher is the intensity of absorption and vice-versa.
This is also called as metal analysis spectroscopy as it is mainly used for the analysis of metals.
Atomic absorption Spectroscopy principle:
The method relies on the principle of absorption spectroscopy.
A liquid sample is allowed to convert into free atoms (desolvated and atomized). These free atoms absorb the light of a specific wavelength. The remaining unabsorbed light is detected and recorded. The intensity of absorption is directly proportional to the concentration of the sample.
AAS Instrumentation:
Atomic absorption spectroscopy has simple instrumentation. But, unlike other spectroscopy methods, it has two additional requirements. These include a specially designed lamp to produce light of a desired wavelength and a burner to prepare the sample for the absorption of light radiation.
Additionally, the instrument also sprays the sample in the solution state over an atomizer (burner). This leads to evaporation of the solvent and leaves a fine dry residue. This residue has neutral atoms in the ground state.
The Instrumentation includes:
1. The atomizer (burner) to dry the sample and produce atoms.
2. Sample container.
3. Fuel and oxidant to burn the sample by heat.
4. Hollow cathode lamp to produce light of the desired wavelength.
5. Detector to detect the absorption intensity.
6. Amplifier and data recorder.
The instrument is available as single and double beam instruments.
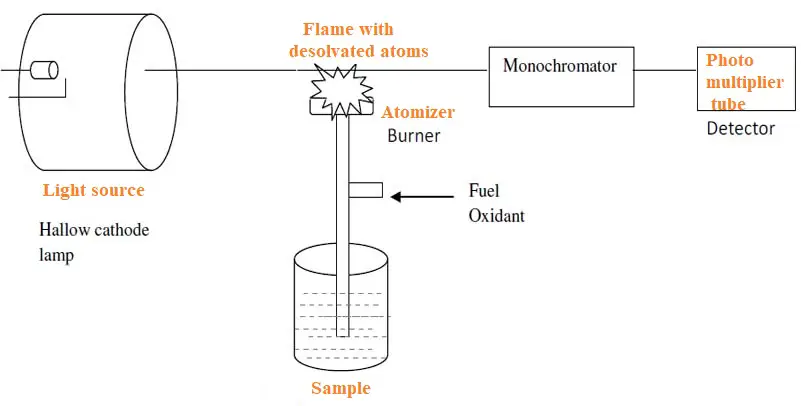
Light source
The light source should produce a narrow spectrum with little background noise. Besides the light should be stable and have sufficient intensity.
Two types of light sources can be used based on the requirement.
1. Hollow cathode lamp
This is most widely used as a light source. Inside the lamp, the cathode is coated with a metal of analyte to be analyzed. For instance, if magnesium is to be analyzed from the sample, a cathode coated with magnesium is used.
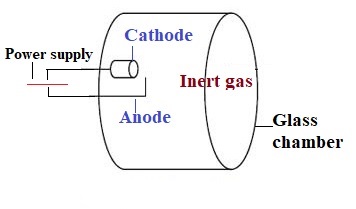
Similarly, for all the other elements like Na, Ca, K, Zn, etc. analysis respective metal-coated cathodes are used in the lamp. The lamp is filled with an inert gas like argon or neon which is ionized by an electric arc. The ions get attracted toward cathodes and strike it leading to excitation of metal ions. This leads to the emission of radiation with a characteristic wavelength of analyte metal.
The advantage of a Hollow cathode lamp is that it provides radiation with a bandwidth of 0.001 to 0.01nm. The use of other methods like monochromators gives radiation with a bandwidth of 1nm. So, these lamps give highly specific radiation.
The disadvantage of this hollow cathode lamp is that for every metal different cathode lamp has to be employed.
2. Electrode-less discharge lamps
These lamps are less conventional in regular use but are essential for the determination of Arsenic and selenium. A bulb containing an element of interest (with argon gas) is present in the lamp. This element is excited using microwave energy or radiofrequency energy
Sample container
This is a beaker-like container of the sample which is placed below the burner preferably. A capillary tube drains the sample to the tip of the burner.
The burner (atomizer)
Here the sample from the capillary rises to the tip of the burner. Here it is burned with the flame. This flame is produced by a fuel and oxidant combination. The sample after evaporation leaves a fine residue of neutral atoms.
Fuel and oxidant
This is a very important part of the entire process to be remembered. If the heat produced is not sufficient then the sample doesn’t form neutral atoms. If the heat of the burner is more, the sample molecules may ionize instead of forming atoms. So both are undesirable for experimentation. Hence a proper combination of fuels and oxidant are to be used to produce recommended temperatures. Commonly used flues include propane, Hydrogen, and acetylene and oxidants are mostly air or oxygen.
Fuel combinations chart
| Fuel combinations | Flame temperature | Metals Analyzed |
| Acetylene + Air | 2550 degrees | For most samples |
| Acetylene + Nitrous oxide | 2900 degrees | Aluminum (Al), Molybdenum (Mo), Silicon (Si), Titanium (Ti) |
| Hydrogen + Air | 2200 degrees | Lead (Pb), Tin (Sn) |
Monochromator
As discussed before, elements have a specific absorption line. But some elements also have secondary absorption lines. Further, there is also emission from the lamp and the flame. Hence, we need to isolate the desired spectral line for the measurement of absorption. To achieve this a monochromator that can filter and provide a resolution of <1nm is employed.
Detector
This part of the instrument detects the intensity of radiation absorbed by the elements. The detector consists of a photomultiplier tube or a simple photocell. The current or potential recorded for the sample absorption is recorded in computer software and then analyzed.
Reading device
This can be a display computer. It displays the absorbance at a specific wavelength.
Atomic absorption spectroscopy procedure:
1. Based on the metal of analysis a suitable cathode lamp is selected.
2. The sample is dissolved in a polar solvent is placed in the container.
3. With the help of fuel and oxidant in the presence of a mixer, the sample solution is sprayed on to the flame.
4. The neutral atoms in the flame absorb light radiation from the cathode lamp. The unabsorbed radiation is recorded by the detector.
Atomic Absorption Spectroscopy applications:
1. Atomic spectroscopy is used for quantitative analysis of metal elements in water, soil, plant material, and ceramics.
2. In health care, it is used to analyze ionic metal elements in blood, saliva, urine samples. The elements analyzed routinely include sodium, potassium, magnesium, calcium, and zinc.
3. To determine heavy metals like iron, manganese, copper, zinc, mercury, lead, nickel, and in urine and blood.
This analysis is essential in case of heavy metal poisoning. Since heavy metal poisoning is mostly lethal a regular monitoring of poison levels in the patient blood is essential.
4. To determine metal elements like copper, nickel, and zinc in the food industry.
5. To estimate Lead in petroleum products.
6. To determine metal concentrations in groundwater and bore well samplings before using for drinking and irrigation.
Helpful for all students. The information is straight to point easily understandable. Thank You..
Nice info. Can phosphorous analysis done by AAS. if not what is the reason and what is used for phosphorous quantification in samples
@VINODHINI, Hi, yes phosphorous can be analyzed with atomic absorption spectroscopy. For this one need to use a phosphorous hollow cathode tube.
Keep it up. Nice and simple information
I have found the information useful and uderstandable.
The Information displayed is very easily and very easily understandable……
this is awesome to have….I passed scientific because of this info
the page is very helpful and found easily understandable
the page was very understandable
THE PAGE WAS USEFUL
The information posted is very easily understandable to readers, possible update about fuel+oxidant combination and interference in aas.
The information posted is short and easy to understand.