Flame photometry is a process wherein the emission of radiation by neutral atoms is measured.
The neutral atoms are obtained by the introduction of the sample into the flame. Hence the name flame photometry.
Since radiation is emitted, it is also called flame emission spectroscopy.
Flame photometer working principle
When a solution of metallic salt is sprayed as fine droplets into a flame. Due to the heat of the flame, the droplets dry leaving a fine residue of salt. This fine residue converts into neutral atoms.
Due to the thermal energy of the flame, the atoms get excited and after that return to the ground state. In this process of return to the ground state, excited atoms emit radiation of a specific wavelength. This wavelength of radiation emitted is specific for every element.
This specificity of the wavelength of light emitted makes it a qualitative aspect. While the intensity of radiation depends on the concentration of the element. This makes it a quantitative aspect.
The process seems to be simple and applicable to all elements. But in practice, only a few elements of Group IA and Group IIA (like Li, Na, k & Ca, Mg) are only analyzed.
The radiation emitted in the process is of a specific wavelength. Like for Sodium (Na) 589nm yellow radiation, Potassium 767nm range radiation.
Flame photometer instrumentation:
1. Burner
2. Monochromators
3. Detectors
4. Recorder and display.
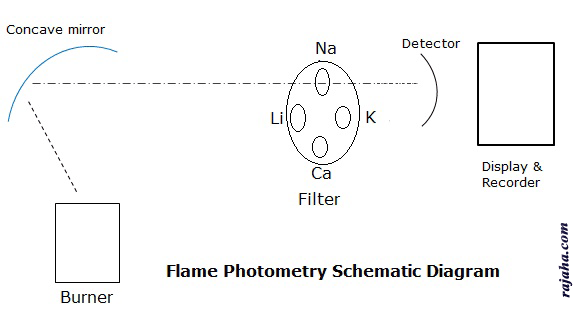
Burner: This is a part that produces excited atoms. Here the sample solution is sprayed into fuel and oxidant combination. A homogenous flame of stable intensity is produced.

There are different types of burners like Total consumption burner, Laminar flow and Mecker burner.
Fuel and oxidants: Fuel and oxidant are required to produce the flame such that the sample converts to neutral atoms and get excited by heat energy.
The temperature of the flame should be stable and also ideal. If the temperature is high, the elements in the sample convert into ions instead of neutral atoms. If it is too low, atoms may not go to the excited state. So a combination of fuel and oxidants is used such that there is the desired temperature.
The following combination of fuel and oxidants are commonly used.
| Fuel | Oxidant | Temperature of Flame |
| Propane + | Air | 2100 Degree C |
| Propane + | Oxygen | 2800 Degree C |
| Hydrogen + | Air | 1900 Degree C |
| Hydrogen + | Oxygen | 2800 Degree C |
| Acetylene + | Air | 2200 Degree C |
| Acetylene + | Oxygen | 3000 Degree C |
Monochromators:
Filters and monochromators are needed to isolate the light of a specific wavelength from the remaining light of the flame. For this simple filters are sufficient as we study only a few elements like Ca, Na, K and Li. So a filter wheel with a filter for each element is taken. When a particular element is analyzed, the particular filter is used so that it filters all other wavelengths.
Detector: A flame photometric detector is similar to that used in spectrophotometry. The emitted radiation is in the visible region, i.e., 400nm to 700nm. Further, the radiation is specific for each element, so simple detectors are sufficient for the purpose of photovoltaic cells, phototubes, etc.
Recorders and display: These are the devices to read out the recording from detectors.
Flame photometer uses/ Applications
1. For qualitative analysis of samples by comparison of spectral emission wavelengths with that of standards.
2. For quantitative analysis to determine the concentration of group IA and IIA elements. For example,
a) Concentration of calcium in hard water.
b) Concentration of Sodium, potassium in Urine
c) Concentration of calcium and other elements in bio-glass and ceramic materials.
Flame photometry limitations:
Unlike other spectroscopy methods, flame photometry finds little use in research and analysis. This is due to
- A limited number of elements can be analyzed.
- The sample requires to be introduced as a solution into fine droplets. Many metallic salts, soil, plant and other compounds are insoluble in common solvents. Hence, they can’t be analyzed by this method.
- Since the sample is volatilized, if a small amount of sample is present, it is tough to analyze by this method. As some of it gets wasted by vaporization.
- Further, during solubilization with solvents, other impurities might mix up with the sample and may lead to errors in the spectra observed.
Thanks a lot because it has been very helpful for my agriculture subject
Thank you so much for the information on flame photometry
Really good slides..need more for analysis and Cognosy point of view i hope u can help me with it
Really nice easy
useful , but it needs References
useful , but it needs References
Nice, easy and like a notes…..very good
Explanation was great I am happy with it.
Thanks for most relevant procedure and data for analysis
By the diagram I can understand easily. .
thank you very much sir
very valuable information .thanks a lot
Good information.
Its very simple and interesting i like it so much
It s easy to understand….
Very good work sir. Thank you.
Thanks for this notes it is helpful to my semester exams
Thank you very much sir.this is very usefull for my general chemistry semester exam
Really easy to understand. .thank you
so easy
Nice explanation it is very helpful
thank you for giving good and easy information.
thanks for easy and good information
thanku so much sir…….
thank you sir ……….
good
really easy thank u 🙂
Thanks a lot because it has been very helpful for my biotechnology semester exam.waiting for more such topics to come !
Good
good work
Very easy to understand. Thank you for this notes.
nice
The uses of burners in flame photometry