Spectroscopy is the measurement and interpretation of electromagnetic radiation that is absorbed or emitted by a sample.
This absorption or emission happens when the atoms of the sample move from one energy state to another in the presence of light.
In other words, it is a science that studies how light interacts with matter.
Light is electromagnetic radiation which is made up of discrete particles called photons.
This light has two characteristics wavelength and frequency.
In short, the wavelength is the distance between two crests or troughs, while frequency is the number of wavelength units passing through a unit of time.
Wavelength is represented by ‘λ’ and the frequency is denoted by ‘ν.’
Natural light is a combination of many spectra. These spectra are the light rays of different wavelengths and frequencies.
The spectra used in spectroscopy vary from ultraviolet, visible, and infrared ranges. The wavelength range for the three spectra is 0-400, 400-700, and above.
When light rays fall on a compound, it gets absorbed to a certain extent, and the remaining is reflected. The wavelength of absorbed light is specific to the material taken.
Spectroscopy is extended to study the substance based on the characteristic absorbance of the above three spectra.
Similarly, at a given wavelength, the intensity of light absorbed is depended on the concentration (quantity) of the substance.
Based on the two phenomena, we try to identify and also measure the quantity of any given substance.
Wherein the absorbance of a specific wavelength of light by the molecules of the sample under test is determined. The more the number of molecules in the sample, the higher the absorbance and vice-versa.
Spectroscopy Principle
Every sample has molecules consisting of some functional groups by which they may incur color or some nature to absorb light of specific wavelengths.
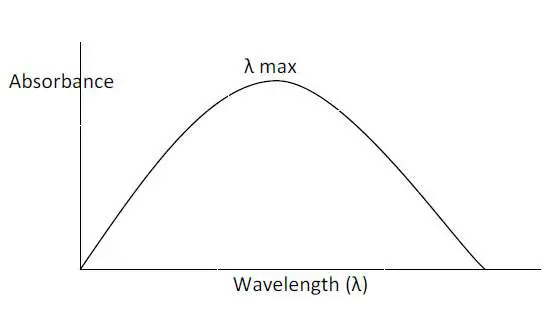
This wavelength at which the sample absorbs to a greater extent is called λ max.
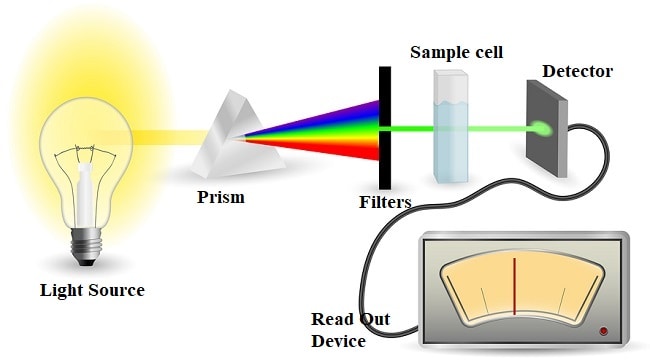
When the light beam is passed on to the sample, the electrons in the molecules absorb energy in the light and go to an excited state.
During this transition, some of the light energy is absorbed while the remaining light falls on the photoelectric detector.
There are different types of spectroscopy based on the technique and use.
Spectroscopy is suitable for both qualitative analysis and quantitative analysis.
For Qualitative spectroscopy
This is the technique to know the type of sample molecule; thereby, one can tell what the sample is and its chemical nature after comparing the obtained analysis curve peaks with that of the standard sample from official books like Pharmacopeias or books on chemical standards, etc.
A sample is subjected to scanning over an entire range of UV or visible radiation.
The point of wavelength where the sample shows maximum absorbance is noted as its λ max.
This λ max is fixed for every sample, and thereby, an unknown sample can be identified by knowing its λ max after comparing it with the standard.
For Quantitative spectroscopy
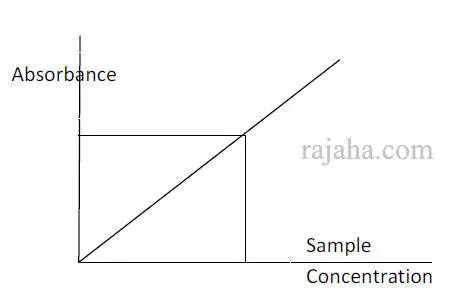
This is a method to determine the exact concentration of a substance in a given sample.
At a specified wavelength (λ max), when a given sample is analyzed by spectroscopy, the concentration in the sample can be known by plotting it against a standard substance graph, as shown in the pic.
For this, a series of dilutions of the standard sample and test sample is taken, and absorbance is measured by spectroscopy.
The absorbance for different concentrations of standard and test are plotted on a graph.
From the absorbance of the test, the concentration of it can be known by extrapolating it on the graph, as shown below in Fig.
UV spectroscopy mechanism
Spectroscopy Applications
- Spectroscopy is the vital detector system in advanced chromatographic methods like HPLC, HPTLC, etc.
- It is also essential as the primary detector system in multi-sample analyzer instruments like Elisa test plate reader, electrophoresis, microplate reader, auto-analyzers, etc.
- It is also a part of continuous culture broths like in fermentation tanks to keep the concentration of microbes or any chemical substance constant. It helps regulate the rate of addition or deletion into the tank.
- Determining biomolecules like corticosteroids, testosterone, aldosterone, etc, is useful.
- It is also helpful in the analysis of phytochemicals like glycosides, tannins, alkaloids, etc.
- It is also helpful in the determination of inorganic substances like Fe, Mg, Ca, Cu, and other salts and their derivatives. Further, oxidative chemicals like potassium permanganate, Ferrous sulfate, etc.