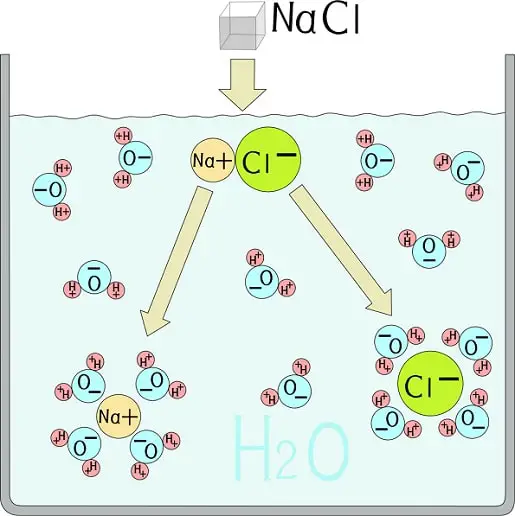
Atomic structure | Models and Experiments in Detail
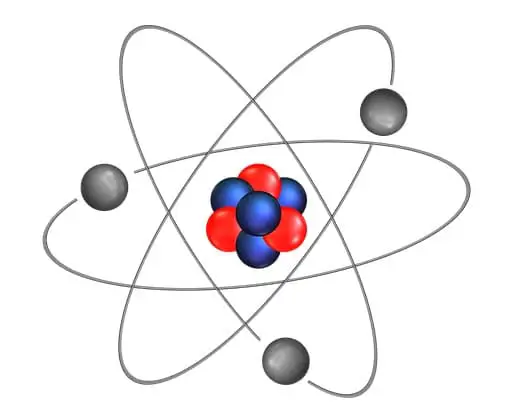
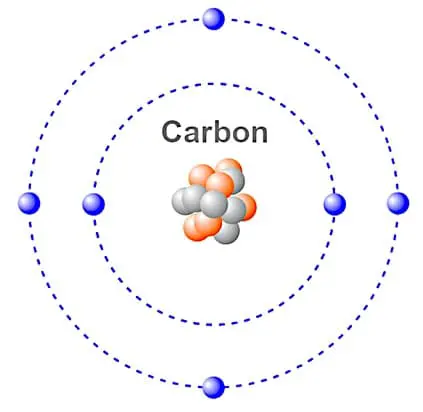
At the initial point of the 19th century, a British chemist John Dalton proposed the atomic theory. This could successfully explain the law of conservation of mass and constant proportion. However, since …